Extreme Climate Survey
Scientific news is collecting questions from readers about how to navigate our planet’s changing climate.
What do you want to know about extreme heat and how it can lead to extreme weather events?
But this is only possible by demystifying the mechanics of cell movement. “We need to understand how cells work if we want to manipulate them,” says Thiam.
This is “undoubtedly an important area of research,” says Clifford Brangwynne, a bioengineer at Princeton University. People sometimes have the misconception that biology is this mysterious universe that somehow operates outside the laws of physics, he says. But the same kinds of physical rules that govern the inanimate world are also at play in living systems.
It is a subject that keeps Thiam’s curiosity curious. Last summer, she submitted three grant applications, and her potential projects could lead in some surprising directions. In one, Thiam proposed collaborating with a biologist who studies the behavior of ants. Yes, insects. This may sound unexpected to someone who studies immune cells.
But Thiam says the cells she studies in the lab — a type of white blood cell called neutrophils that seek out and destroy dangerous microbes — have something in common with ants. Nor do they have a central control system that tells them how to do their jobs. Instead, the first wave of ant hunters find a food source and then leave a chemical bread trail for other ants to follow. Similarly, neutrophils leave a chemical trail for their reinforcements. This type of collective behavior, where interactions between individuals influence group action, has been well studied in ants, Thiam says. “We can learn a lot from this.”
Now, she wants to know if what a neutrophil ends up doing with a microbe it detects—eat it, poison it, trap it—affects the searching behavior of subsequent cells.
That undercurrent of wanting to learn and ask questions—about science and herself—has flowed throughout her career. Thiam grew up in Senegal and moved to France for her university degree and diploma. Her Ph.D. the work revolved around understanding how the nucleus affects a cell’s ability to move. At the time, conventional wisdom on cell migration largely ignored the nucleus, Thiam says. Scientists thought that crawling cells took three basic steps. They extend a “leg”, attach it to a nearby surface, then pull back, pulling the cell body forward. (Imagine Batman scaling the side of a building with his grappling hook.)
But this largely two-dimensional view overlooked the cells’ 3-D environment, Thiam says. Sure, cells can crawl along flat surfaces, but what about when they need to crowd into tight spots? From experiments that had cells moving through ever-smaller pores, Thiam’s team reported Nature Communications in 2016, the nucleus helps determine whether immune cells can migrate in confined environments. Compare the movement of the cell to passing a plastic bag through a small hole. If the bag contains a kiwi, it probably won’t fit.
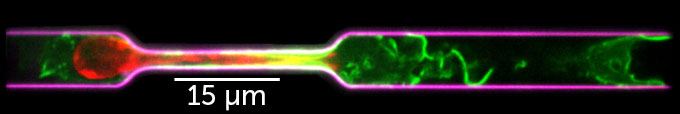
Thiam and her colleagues found that cells have a way of warping their nuclei, up to a point. The cells rupture the membrane surrounding the nucleus and pull out some of its guts, making the whole thing more able to flow through a constriction. It’s like crushing that kiwi until the gooey skin breaks and the fruit is wobbly and not firm. Now I can squeeze through a smaller space. Until this work, no one had shown that nuclei behave like this, says Thiam.
Later, during her postdoctoral work in Clare Waterman’s lab at the National Institutes of Health in Bethesda, Md., Thiam continued her investigations of cell nuclei. In a 2020 paper in Proceedings of the National Academy of Sciencesshe and her colleagues reported on one of the strangest aspects of biology, a cellular defense mechanism called NETosis. It is a way for neutrophils to physically block invading bacteria, fungi or viruses. Pathogens crawl into the body and – BOOM – suddenly they are trapped, like dolphins caught in a fishing net. But this mesh is made of DNA: the immune cell breaks down its own genetic material to catch the germs. “It’s this crazy phenomenon,” says Thiam.
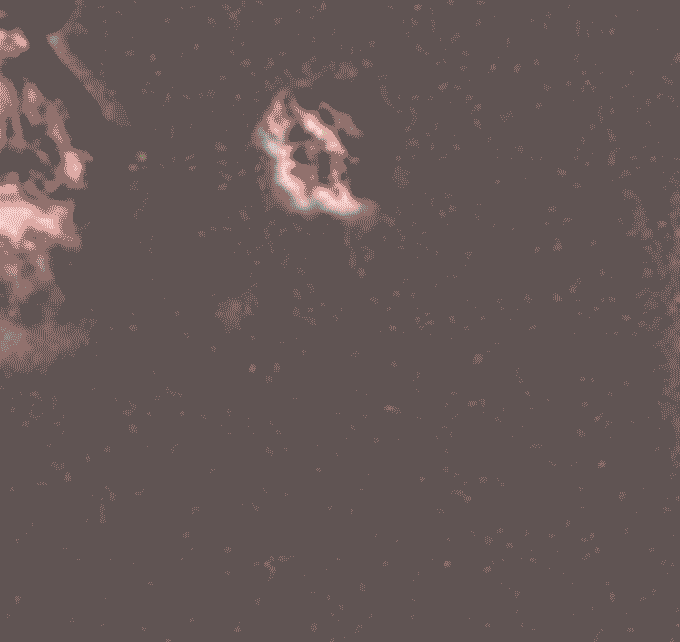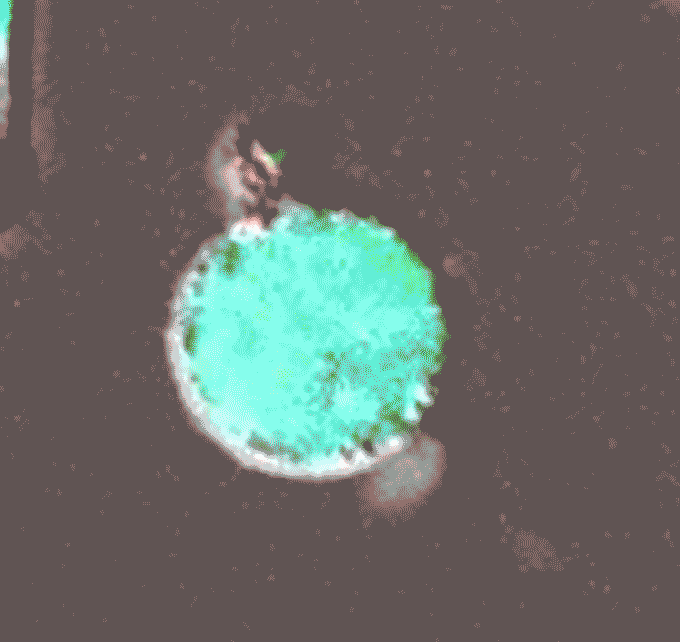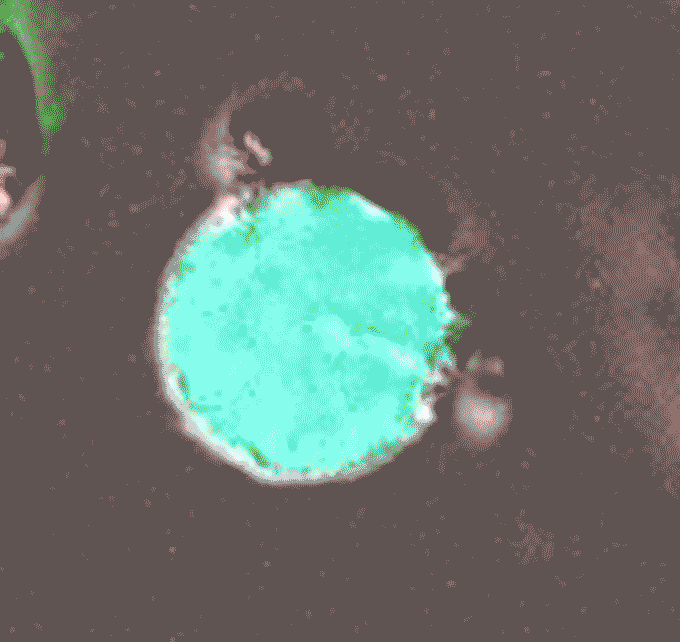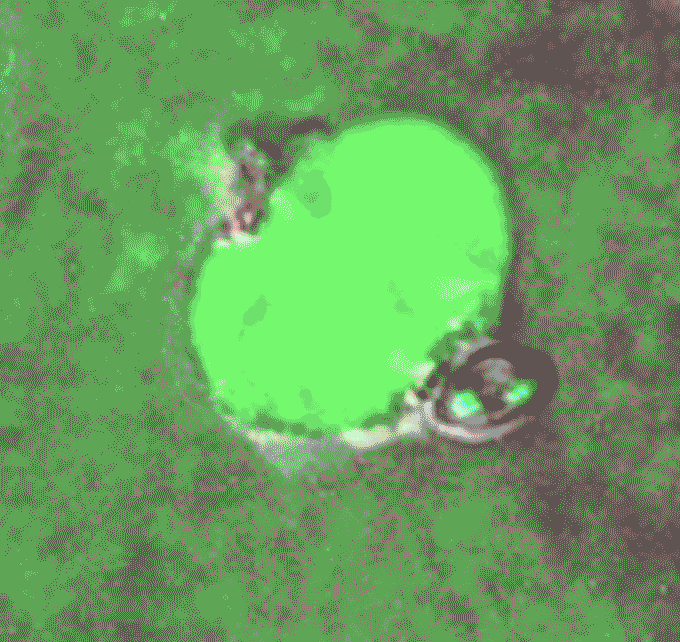
Scientists had first reported NETosis in 2004, but they didn’t really know how it worked. This is what Thiam’s team took on. Using state-of-the-art microscopy and gene-editing techniques, researchers describe the sequence of events that begin with a cell’s DNA packaged inside the nucleus and end with it bulging outside the cell.
“She has this fearlessness to tackle these really, really challenging problems in cell biology,” says Brangwynne, who is also a Howard Hughes Medical Institute investigator. He thinks the fear stems from her background. Thiam has crossed borders between nations, different fields of science and languages (she speaks four). “I think she’s really not afraid of anything,” he says.
But Thiam says she still wonders if she’s smart enough, works hard enough and is capable of being a good scientist and mentor. “I think it’s okay to have doubts,” says Thiam. She accepts them, tries not to let them defeat her, and thinks about how she can improve. And every time Thiam wonders, she tries to remember that she and others believe in her—and then she gets on with her work, writing grants, doing science, and training students. “I just try to keep pushing,” she says.
#biophysicists #work #day #doctors #control #immune #cells
Image Source : www.sciencenews.org